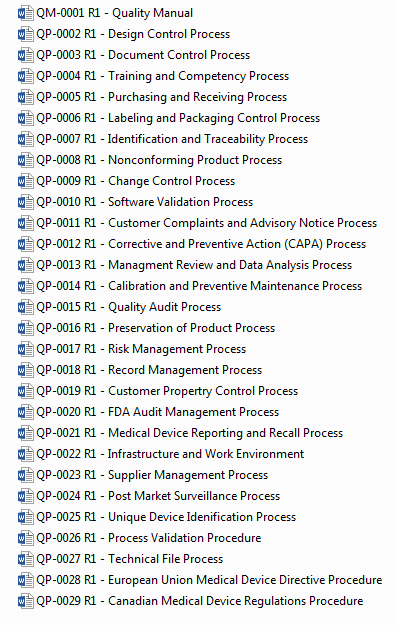
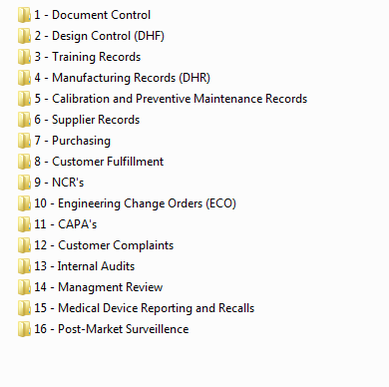
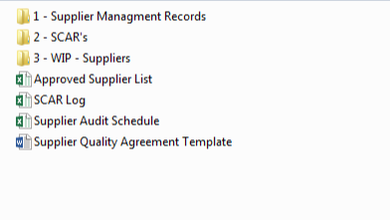
Medical Device Quality System Templates
Quality Systems for Medical Device Startups and Small Businesses
- FDA QSR Compliant
- EU and Canadian MDR Compliant
- ISO 13485:2016
- ISO 14971 - Risk Management
- Ready to Implement
- Provided in Editable Format
100% Satisfaction Guarantee
If you are not satisfied for any reason, Med Dev QMS will refund your entire purchase price!
If you are not satisfied for any reason, Med Dev QMS will refund your entire purchase price!